Services
We focus on building bespoke, interpretable, predictive models for the life science industry. Full list of services include:
Predictive modelling
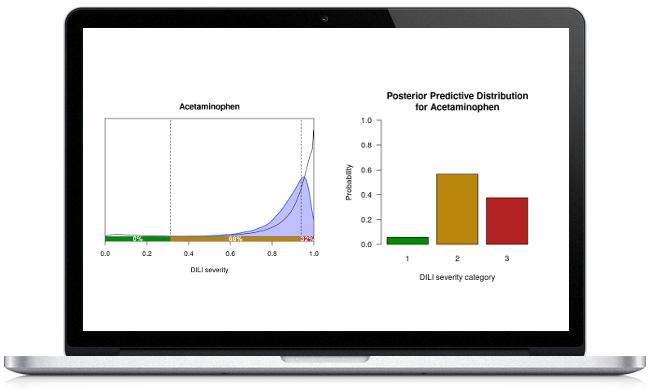
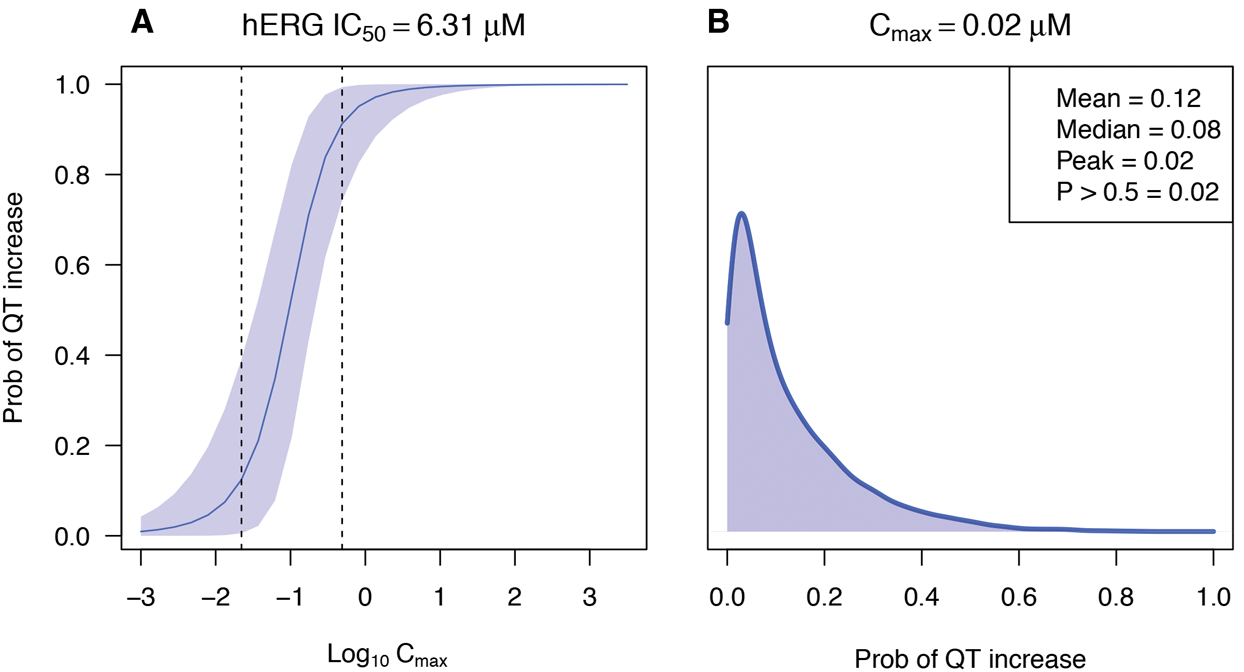
Safety pharmacology, toxicology, compound ranking/prioritisation, assay optimisation.
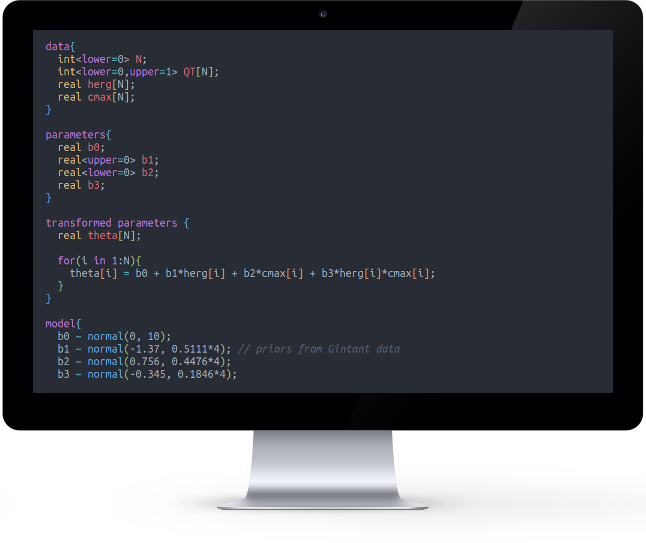
Bayesian statistics
Statistical inference for clinical and pre-clinical experiments, mainly from a Bayesian perspective.
Design of Experiments
Maximising the information gained from experiments, avoiding confounding, and obtaining generalisable results.
Automating analytical workflows
Save time, reduce error, increase reproducibility.
Regulatory writing
Specialising in clinical regulatory writing and submissions.